Early Detection of Critical Congenital Heart Defects
Hadya LAGGOUN
Congenital Heart Diseases (CHD) are one of the leading causes of infant mortality. About one in every four newborns with a heart defect has critical CHD which are usually defined as structural malformations of the heart for which neonates require surgery or catheter-based intervention in the first year of life. Symptomatic newborns with critical heart lesions are usually identified soon after birth, while others/asymptomatic are not diagnosed until after discharge from the birth hospitalization. Although challenging, the importance of an accurate and early diagnosis with a prompt effective care in improving survival, short and long-term outcomes in infants with such defects is undeniable. Indeed, the risk of morbidity and mortality is extremely high when there is a delay in diagnosis and timely referral to a cardiac center with expertise in treating these patients.
In this topic, we propose to review the different methods used to early detection of critical CHD. Particular attention shall be paid to combined pulse oximetry and clinical examination approach in asymptomatic newborns as it is within the reach of any clinician and can prove crucial in deciding newborn’s fate, wether it is a healthy life, a life with disability or even death.
Overview of Critical CHD1, 2
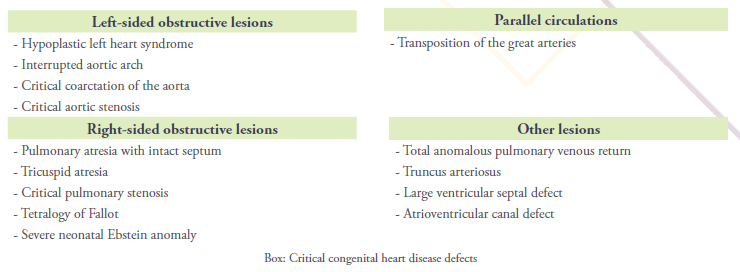
There are several types of ductal-dependent CHD and cyanotic lesions that are included in critical CHD (Box).
Ductal-dependent congenital heart lesions are dependent upon a patent ductus arteriosus (PDA) (cf. Sari T. Le Changement pour la Vie. Medpress. 2020;5)3 which is necessary to supply pulmonary blood flow retrogradely from the aorta in patients with right-sided obstructive lesions, systemic circulation in those with left-sided obstructive lesions, or to allow adequate mixing between parallel circulations (eg, transposition of the great arteries (TGA)). Tetralogy of Fallot (TOF) (cf. Laboudi A. La Tétralogie de Fallot – La Maladie Bleue. Medpress. 2020;5)4 and tricuspid atresia may or may not be ductal-dependent depending upon the degree of right ventricular outflow tract obstruction and the presence and size of a ventricular septal defect (VSD) in tricuspid atresia.
Closure of the PDA in the first days of life can precipitate profound cyanosis. Therefore, a rapid initiation of prostaglandin E1 to reopen and maintain the patency of the ductus arteriosus is vitally important. Mechanisms of cyanosis according to each defect are described in Cardiac assessement section below. Many, but not all, cyanotic congenital heart defects are ductal-dependent; total anomalous pulmonary venous connection (TAPVC), truncus arteriosus (TAC) are non-ductal-dependent CHD that cause cyanosis.
Of the defects listed in Box, some forms of CHD may not require surgery in the neonatal period but still require intervention in the first year of life, such as a large VSD or an atrioventricular septal defect.
Depending on the type of heart lesion, patients with CHD may present with antenatal cardiac ultrasound diagnosis, or postnatal detection of a heart murmur, heart failure, shock and/or cyanosis.
Ideally, in mothers with adequate prenatal care, many critical CHD can be diagnosed prenatally after abnormalities detected on obstetric ultrasonography prompt a fetal echocardiogram to confirm the diagnosis. If a prenatal diagnosis is not achieved, early detection of critical CHD postnatally may be helpful to improve overall outcomes given the window of time for potential intervention before an infant develops signs of compromise.
Antenatal Diagnosis of Critical CHD5, 6
Prenatal screening has become a significant contributor to early identification of critical CHD and retains an important role in reducing mortality related to it. Indeed, studies evaluating the effect of prenatal diagnosis of critical CHD on neonatal mortality concluded that in patients with comparable anatomy, standard risk and optimal care, newborns with a prenatal diagnosis were significantly less likely to die prior to planned cardiac surgery than those with a comparable postnatal diagnosis.7
In order for a prenatal diagnosis to occur, there must be adequate and timely prenatal care.
Besides, evidence shows that there are certain critical CHD lesions that are much more likely to be diagnosed prenatally than others. A 4-chamber prenatal ultrasonographic view of the heart alone may miss several forms of critical CHD, because, in many forms, the 4-chamber heart view is within normal limits. Therefore, there is growing evidence demonstrating that basic cardiac assessment should include views of the outflow tracts in addition to the 4-chamber view as a part of every second trimester fetal evaluation.9 This may enhance detection rates and increase the likelihood of identifying conotruncal anomalies such as TOF, TGA and truncus arteriosus. However, type of ultrasound practice, operator training and experience, gestational age, maternal weight, fetal position and type of defect are shown to be important determinants of the test’s sensitivity.9
Conversely, fetal echocardiography provides more detailed evaluation of fetal cardiovascular structure and function than a basic ultrasound examination. But, as a less cost-effective screening approach, a fetal, maternal or familial risk factor for CHD must be identified to prompt referral for fetal echocardiography (you can consult the common indications for referral for fetal echocardiogram suggested by the American Heart Association on this statement).10
Once a fetal cardiac abnormality is detected, additional genetic and extra-cardiac evaluation and follow-up (which are beyond the scope of this topic) are indicated. Moreover, delivery should be planned to ensure an appropriate level of care for the mother and newborn.
Even with fetal echocardiograms, which are not available universally, the diagnosis of critical CHD during pregnancy remains complex: coarctation of the aorta (COA) is particularly difficult to accurately diagnose prenatally.11 (cf. Ayadi K. Coarctation Aortique. Medpress. 2020;5).12
Postnatal Diagnosis of Critical CHD1,6
In infants with critical CHD, the timing of clinical presentation differs according to the underlying lesion and its dependence upon a PDA.
In fact, during the birth hospitalization, clinicians may be confronted with serious and life-threatening manifestations including cyanosis, respiratory distress and rapid progression to advanced states of shock, that lead to the suspicion of critical CHD. This is all the more true in patients with ductal-dependent lesions as closure of the PDA within the first few days of life precipitates rapid clinical deterioration; infants with these lesions may present with cardiogenic shock as the ductus arterious closes and systemic perfusion decreases. Cardiomegaly and lack of response to volume resuscitation help differentiate a shock of cardiac origin from other causes of shock (eg. sepsis). For these patients, urgent interventions are necessary to reopen or maintain patency of the ductus arteriosus (ie, prostaglandin therapy) and thus decrease risk of death and significant morbidity.
However, early detection of critical CHD remains challenging in many neonates because clinical findings may be subtle or absent appearing only after discharge; the risk of mortality is substantially higher for the latter.
Prior to the initiation of routine pulse oximetry screening (discussed below), the newborn examination alone fails to detect more than half of infants with heart disease;13 the most commonly delayed diagnoses included COA, interrupted aortic arch, aortic stenosis, hypoplastic left heart syndrome (HLHS), TGA, pulmonary valve stenosis and TOF.
Ultimately, a thorough review of the history and physical examination of each neonate (regardless of symptoms) remains imperative and should be accomplished with attention to findings suggestive of critical CHD. Combined to physical examination, pulse oximetry improves the identification of asymptomatic patients with critical CHD.
Otherwise, the extent of evaluation that should be performed during the birth hospitalization is determined by the presence or absence of symptoms. If CHD is clinically suspected in a patient, echocardiography provides a definitive diagnosis with information on cardiac anatomy and function.
History:14,15
The health status of newborns is directly connected with pregnancy and delivery; thus, the first step in the assessment of the newborn’s cardiovascular system is a careful review of maternal, fetal and neonatal conditions that are associated with an increased risk of CHD.
There is an overall threefold increased risk for CHD when a first-degree relative has CHD. This risk is also two- to threefold higher in preterm (gestational age < 37 weeks) compared with term infants.
Maternal diabetes mellitus, uncontrolled phenylketonuria, congenital infections (eg, rubella), maternal influenza or flu-like illness during pregnancy should increase the vigilance of the clinician for the possibility of CHD in the newborn. In addition, drugs taken during pregnancy (eg, phenytoin and retinoic acid) as well as smoking and/or alcohol use can be associated with cardiac defects.
Last but not least, genetic causes are increasingly recognized in the etiologies of CHD and many genetic syndromes are associated with a heightened risk of CHD (eg, Down syndrome, DiGeorge syndrome…).
The presence of any of the previous factors should raise the index of suspicion, but a complete physical examination should be performed regardless.
Cardiac assessment:1,16
After a general assessment (including weight), cardiac evaluation of the newborn requires great skill with the techniques of inspection, palpation and auscultation. Because changes from the placental circuitry to the newborn lung circuitry occur over the first few hours, days and weeks of life, cardiovascular assessments should be done shortly after birth, at 6 to 12 hours of age, and again at 1 to 3 days of life. Clinicians should also be alert to clinical manifestations of CHD that may be detected in the course of initial routine newborn visits because some neonates with critical CHD are asymptomatic during the birth hospitalization and then develop signs and symptoms after discharge, typically by 2 weeks of age.
Inspection: when examining a newborn, clinicians should start by closely observing the baby. The presence or absence of cyanosis, obvious congenital malformations, the general activity of the neonate, and breathing patterns are all important.
Cyanosis is an important sign of critical CHD which may be visible with 3 to 5 gm/dL of reduced hemoglobin. The fact that subjective visual appreciation of cyanosis is often difficult in darkly pigmented infants, patients with mild desaturation or anemia, reinforces the role of pulse oximetry (see Pulse oximetry screening below).
If cyanosis is present, one must differentiate between peripheral and central cyanosis.
Central cyanosis or bluish discoloration of the tongue and mucous membranes caused by desaturation of arterial blood indicates cardiac and/or respiratory dysfunction and is always abnormal. Cyanosis caused by pulmonary disease is often responsive to the administration of oxygen contrary to central cyanosis caused by critical CHD which does not change significantly when patients are placed in an oxygen-enriched environment.
In patients with critically obstructive right heart lesions, progressively severe cyanosis occurs as the ductus closes and blood flow to the lungs decreases. In those with critically obstructive left heart lesions, adequate atrial septal communication and a patent ductus, they typically exhibit only minimal desaturation. However, upon closure of the ductus, systemic circulation is compromised, resulting in poor peripheral perfusion and cyanosis. Even with ductal patency, a restrictive atrial communication also results in decreased shunting of the oxygenated pulmonary venous return into the right heart, severe pulmonary edema, and pulmonary hypertension, all of which contribute to decreased systemic oxygenation. In a similar manner, patients with parallel pulmonary and systemic circulations depend upon the PDA and atrial communications for mixing of oxygenated and deoxygenated blood. With ductal closure in the absence of an adequate atrial septal defect, profound cyanosis ensues.
On the other hand, circumoral cyanosis or bluish discoloration around the mouth, which is associated with nipple or breast feeding, should normally resolve following the feeding.
Peripheral cyanosis or bluish discoloration of hands and feet, also known as acrocyanosis, does not involve the mucous membranes and usually reflects benign vasomotor changes in the diffuse venous structures in the affected areas. It does not indicate pathology unless cardiac output is extremely low, resulting in cutaneous vasoconstriction.
In addition, mottling or pallor can be a sign of diminished cardiac output as blood is shunted away from the skin to support more central organs and tissues.
It is important to pay attention to dysmorphic signs as they may suggest a syndrome associated with heart malformation.
In affected newborns with late presentation, parents most commonly report difficulty with feeding, unexplained irritability, excessive sleeping or decreased activity and excessive sweating that is increased with feeding and may occur during sleep.
Infants may also have respiratory distress that is reported by parents as fast or hard breathing, worse with feedings, or a persistent cough or wheeze. Normal babies breathe about 40 to 60 times a minute. Tachypnea and other respiratory symptoms can occur due to pulmonary edema from a rapid, massive increase in pulmonary blood flow as pulmonary vascular resistance falls shortly after delivery; or due to pulmonary overcirculation from left to right shunting.
Palpation: a complete cardiac examination of a newborn includes the determination of capillary refill time (CRT) as well as palpation of precordium, peripheral arterial pulses and the abdomen.
In newborns, when light pressure is applied on the skin or nail beds, normal color should return within 3 to 4 seconds after the pressure is released (CRT).
Precordial palpation must be performed for each newborn. It determines whether the heart is normally located, helps noting any hyperactivity suggestive of ventricular volume or pressure overload and checking for thrill.
Measurement of upper and lower extremity blood pressure and assessment of peripheral pulses are particularly important. Clinicians should evaluate the carotid, brachial, femoral and pedal pulses to detect differences between sides and upper and lower extremities. The diagnosis of COA or interrupted aortic arch is strongly suggested in newborns with decreased or absent pulses in the lower extremities with strong upper extremity pulses. If pulses are unequal, four extremity blood pressures are obtained while ensuring that the newborn is in the same state during all measurements. Cuff size is also critical: if too narrow, it gives falsely high readings, and if too large a cuff may yield low readings. Higher blood pressure in the arms than legs may be caused by COA.
Finally, palpation of the abdomen serves to determine the size, consistency, and location of the liver. Hepatomegaly is a non-specific finding that may be seen in infants with heart failure and increased central venous pressure.
Auscultation: auscultation is the main focus of the exam. Heart rate, rhythm and heart sounds (especially murmurs) must be assessed systematically and sequentially. The dynamic properties of the newborn heart make this assessment more difficult than the cardiac assessment of an adult; thus, effective auscultation of the neonatal heart requires much practice over time.
For neonates up to 6 days of age, the normal range of heart rate is 90 to 160 beats per minute.
The second heart sound (S2), which corresponds to the closure of the aortic and pulmonary valves, must be given particular attention.
Evaluation of the splitting of S2 is of great importance. In healthy newborns, S2 splits physiologically with inspiration and becomes single during expiration. It is important to note that, due to newborn’s rapid heart rate, detection of S2 splitting may be challenging.
In general, CHD are often accompanied by a pathological S2. A single S2 occurs in aortic atresia, pulmonary atresia (PA), TAC, severe pulmonary stenosis, TOF, and conditions with pulmonary hypertension. In TGA, the pulmonary artery is located posterior and directly behind the aorta; thus, the softer pulmonary component of the S2 is often inaudible.
A widely or fixed split S2 occurs with atrial septal defect and other lesions associated with right ventricular volume overload or right-sided conduction delays.
On the other hand, the presence of a heart murmur during the first days of life is rarely pathognomonic: a cardiac murmur is not always the sign of heart disease, and its absence doesn’t assure normality.
The absence of a murmur despite a congenital cardiac abnormality can be due to lack of high velocity of turbulent blood flow able to generate a murmur (ie, HLHS, TGA, TAPVC, PA); or to decreased ventricular function in aortic stenosis.
Murmurs associated with heart disease can be distinguished from innocent murmurs (caused by the transition of fetal to neonatal circulation) based upon the intensity, location, quality of the murmur and associated findings.
Pulse oximetry screening:6, 17, 18
Prior to institution of routine pulse oximetry screening, approximately one-quarter of infants with critical CHD were not diagnosed during the birth hospitalization. Newborns screening using pulse oximetry has been introduced as an adjunct to newborn physical examination to help early detection of critical CHD. It has been added to the list of Recommended Uniform Screening Panels (RUSP) since 2011, and advocated by several health-care authorities. All have mandated that pulse oximetry screening for critical CHD be completed before a newborn is discharged from the hospital after birth.
Pulse oximetry is easily accessible, inexpensive and noninvasive and can be performed by the nurses at the newborn’s bedside. Based on the existing literature, the cost-effectiveness of pulse oximetry screening for identifying critical CHD has been demonstrated.
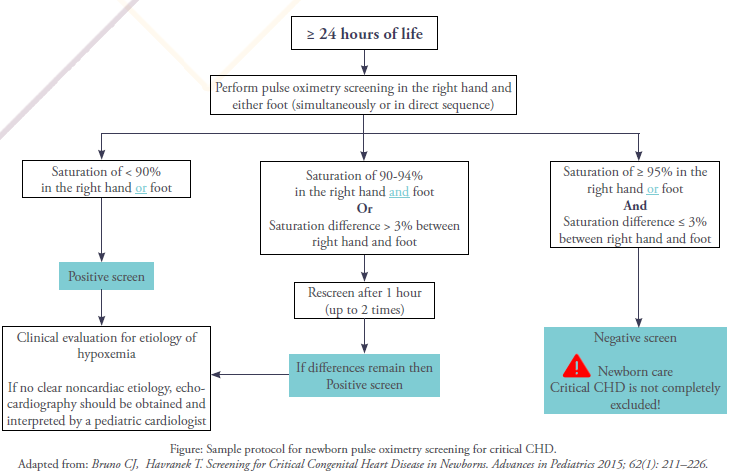
A sample basic pulse oximetry screening protocol is represented in the Figure. The general principle is that a healthy newborn infant should have a pulse oximetry reading ≥ 95% after 24 hours of life (negative screen). Screening is recommended to be delayed until after 24 hours of life to avoid false-positive results, because hypoxemia commonly occurs during the transition from intrauterine to extrauterine life conditions.3
Oxygen saturation (SpO2) is measured in the right hand (preductal) and either foot (post ductal) using a motion-tolerant pulse oximeter. It is preferable to use the right hand rather than left because the left subclavian artery arises close to the ductus arteriosus, and some of its flow may come from the ductus and thus not accurately reflect preductal values.
Saturations less than 95% should rise concern. A newborn with a pulse oximetry reading of less than 90% in either extremity warrants an immediate evaluation to identify the cause of hypoxemia (positive screen). A newborn with a pulse oximetry reading of 90% to 94% in both upper and lower extremities may be screened for up to 2 hours to determine if these differences are real or transient. In addition, any difference in upper and lower extremity oxygen saturations greater than 3% also warrants further evaluation.
It is worth noting that pulse oximetry allows the identification of conditions of hypoxemia other than critical CHD including noncritical cardiac defects, infections and pulmonary/respiratory disorders. Although detection of these conditions is currently considered as false positives, it is important to identify early these potentially fatal but treatable pathologies.
Finally, there are several limitations to pulse oximetry screening for critical CHD in neonates. The most important one is that negative screening with pulse oximetry can not «rule out» the presence of a critical CHD. In fact, pulse oximetry in newborns may miss as many cases of critical CHD as it detects; hence, clinicians must be trained about these potential patients with false-negative results, so that other clinical findings of critical CHD are not ignored. If there is clinical suspicion for critical CHD, additional evaluation should be pursued even in the setting of a normal pulse oximetry result.
To sum up, since timing of diagnosis affects mortality in critical CHD, a precise diagnostic approach and a meticulous attention to every aspect of care are absolutely essential for providing a positive outcome and quality of life to infants with such diseases. Although the use of fetal cardiac ultrasonography in screening for critical CHD, some defects can be challenging to identify. Similarly, attempting to detect critical CHD by newborn physical examination alone has its limitations. Therefore, pulse oximetry in addition to a careful neonatal physical examination present optimal operative characteristics that can enhance the clinician’s ability to detect life-threatening CHD in a timely manner, especially in low and middle-income countries where technology medical is not entirely available.
References
1- Altman CA. Identifying newborns with critical congenital heart disease. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. https://www.uptodate.com.
2- Lissauer T, Carroll W. Illustrated Textbook of Paediatrics. Fifth edition. (2018).Chapter 18: Cardiac disorders; pp 320-343.
3- Sari T. Le Changement pour la Vie. Medpress. 2020;5.
4- Laboudi A. La Tétralogie de Fallot – La Maladie Bleue. Medpress. 2020;5.
5- Copel J.Fetal cardiac abnormalities: Screening, evaluation, and pregnancy management. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. https://www.uptodate.com.
6- Bruno CJ, Havranek T. Screening for Critical Congenital Heart Disease in Newborns. Advances in Pediatrics 2015; 62(1): 211–226.
7- Holland BJ, Myers JA, Woods CR Jr. Prenatal diagnosis of critical congenital heart disease reduces risk of death from cardiovascular compromise prior to planned neonatal cardiac surgery: a meta-analysis. Ultrasound Obstet Gynecol 2015; 45:631.
8- International Society of Ultrasound in Obstetrics and Gynecology, Carvalho JS, Allan LD, et al. ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol 2013; 41:348.
9- Wong SF, Chan FY, Cincotta RB, et al. Factors influencing the prenatal detection of structural congenital heart diseases. Ultrasound Obstet Gynecol 2003; 21:19.
10- Donofrio MT, Moon-Grady AJ, Hornberger LK, et al. Diagnosis and Treatment of Fetal Cardiac Disease: A Scientific Statement From the American Heart Association. Circulation 2014;129:2183–2242.
11- Buyens A, Gyselaers W, Coumans A, et al. Difficult prenatal diagnosis: fetal coarctation. Facts Views Vis Obgyn 2012; 4(4): 230–236.
12- Ayadi K. Coarctation Aortique. Medpress. 2020;5.
13- Wren C, Richmond S, Donaldson L. Presentation of congenital heart disease in infancy: implications for routine examination. Archives of Disease in Childhood – Fetal and Neonatal Edition1999; 80(1): F49–F53.
14- Jenkins KJ, Correa A, Feinstein JA, et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 2007; 115 (23):2995-3014.
15- Pierpont ME, Basson CT, Benson DW Jr, et al. Genetic basis for congenital heart defects: current knowledge. Circulation 2007; 115 (23):3015-38.
16- Fillipps DJ, Bucciarelli RL. Cardiac Evaluation of the Newborn. Pediatric Clinics of North America 2015; 62(2):471-489.
17- Oster M. Newborn screening for critical congenital heart disease using pulse oximetry. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. https://www.uptodate.com.
18- Chamsi-Pasha MA, Chamsi-Pasha H. Critical congenital heart disease screening. Avicenna J Med 2016; 6(3): 65–68.