Treg-based Cellular Immunotherapy Origins and Future Prospects
Rihab FELLAH
Lymphocytes T have immerged in the last decades as being a very diverse lineage of immune cells. Their diversity lies in the different functions that numerous subtypes have. These functions can be intricate and sometimes overlapping making them very hard to study and characterize. But thanks to animal models, modern bioengineering, molecular biology and also human pathology (consequent to the lack of certain subtypes), immense progress has been achieved in this field. Today we chose to discuss an ingenious sybtype of these cells: regulatory T lymphocytes or Tregs. Not only have they been the center of multiple research studies, their natural tendancy towards immunomodulations makes them the target of many ongoing therapeutic clinical trials.
What is a Treg cell?
It is a lymphocyte T of CD4+ or CD8+ lineage whose main function is to suppress any type of immune response that is unnecessary or damaging or that targets self-antigens.
In fact, early in the immune development, T progenitors, after migration to the thymus, undergo a “Negative Selection”. In this process, LTs’ affinity towards self-antigen (i.e. autoreactivity) is tested, depending on the results, these cells will have three outcomes: (1) For those having high affinity towards self-antigens, apoptosis is inevitable (R.I.P). (2) Others having low affinity for self-antigens will migrate to peripheral lymphoid tissue (i.e. lymph nodes) to become functional defenders of the organism. (3) The final group, whose affinity towards self-antigens is intermediate, will be converted into Tregs. This happens through upregulation of pathway proteins that will activate a transcriptional factor called Forkhead box P3 (a.k.a Fox p3). This transcriptional factor is responsible for the modulatory abilities of Tregs.
Once out of the thymus our new immunomodulators are said to be native or thymic Tregs (nTregs or tTregs). The acquisition of this regulatory function isn’t limited to thymic LTs. Indeed, naïve LTs can be induced peripherally or ex-vivo in certain cytokine environments to express Fox P3. They would be called induced Tregs or peripheral (iTregs, pTregs).
Whether these induced cells are functionally stable or not is a question that scientists had to answer by defining Treg’s characteristic phenotype which is the combination of the following conditions: a suppressive function, an expression of Fox p3 and a development favoured by certain key factors (mainly IL2, TGFb).
Fox P3 maintains its role as the most specific marker of the regulatory function of a T cell, however other immune cells can transiently express it, which means that its presence isn’t synonymous of a cell’s commitment to Treg lineage.
Interestingly, some iTregs can convert back to effector T cells by losing Fox P3 expression. These exTregs are functionally unstable and lack necessary commitment. A reliable proof of such a commitment is the demethylation of Fox P3 promoter which confers stability to this transcriptional factor’s expression.
How is this useful in therapeutic practice? When transplanting Tregs scientists isolate LTs from peripheral blood. Once isolated, they have to filter them in order to obtain pure Tregs. In this case, Fox P3 is used as a marker. And here comes the problem of stability. What if the isolated Fox P3 positive T cells weren’t stable Tregs? What if they only expressed Fox P3 (and therefore acted as modulatory cells) for a period of time and then went back to being the effector T cells they were? The question of Treg stability is still an issue that’s being continuously researched and studied. We will talk about the difficulties of transplanting Tregs further on, among which Treg stability is a leading one.
How do Tregs work?
It is thought that Tregs modulate responses against self-an
Proof of this has been demonstrated in the study of IPEX: Immune
Furthermore, supporting this theory are data from animal studies conducted on a certain type of mice called NOD mice (Non-Obese Diabetic mice) that are prone to developing auto-immune diabetes mellitus. It was observed that transferring Tregs from other mice types could stop and even reverse the course of the disease, demonstrating thus the immunosuppressive quality of the Tregs.
Indeed, molecular studies later confirmed the powerful regulatory and immunosuppressive capacities of Tregs. This is achieved through various mechanisms.
Among the main mechanisms of action of Tregs, they can elicit a state of “Starvation/theft of IL2”. As these cells highly express the CD25 (alpha chain of the IL2 receptor), they can act
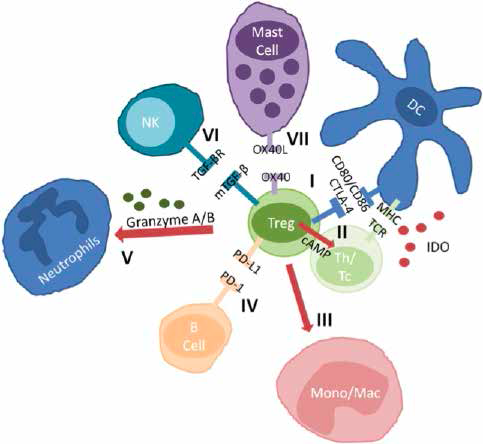
Other mechanisms include activation of immune checkpoints CTLA-4, PD1L, inducing changes in local immune environments. The latter consists of secreting soluble regulatory factors like IDO (Indoleamine 2,3 DiOxygenase) and TGFb (Transforming growth factor- b); these result in inhibition of both adaptive and innate immune cells, apoptosis of professional phagocytes, inhibition of degranulation of mast cells as well as the pro-inflammatory signalling of antigen presenting cells (APCs). (
Fig 1. Chosen mechanisms used by T regulatory cells (Tregs). I suppression of antigen presentation, induction of expression of IDO in DCs via the CTLA-4; II inhibition of activation of Th and cytotoxic T effector via cell-to-cell interactions, extracellularly produced ade- nosine via CD39, CD73 receptors; transferred cAMP and consumption of IL2; III induction of apoptosis of mono/mac; IV inhibition of B-cell proliferation and induction of apoptosis via PD-1; V induction of apoptosis of neutrophils; VI inhibition of function and prolife- ration of NK cells; VII inhibition of degranulation of mast cells. cAMP cyclic adenosine monophosphate, CD cluster of differentiation, DCs dendritic cells, IDO indoleamine 2,3dioxygenase, IL interleukin, mono/mac monocytes/macrophages; NK natural killer, PD-1 programmed cell death-1, Tc cytotoxic T effector, Th T helper.
Reproduced from Gliwiński & al. Cell-based therapies with T regulatory cells. BioDrugs (2017) 31:335–47.10.1007/s40259-017-0228-3
How can Tregs be used in therapy?
As previously illustrated, auto-immunity and deleterious inflammatory diseases can be caused by absent or dysfunctional Tregs as seen in type-1 diabetes mellitus (T1DM), Multiple sclerosis (MS) or Crohn’s disease (CD).
In either Hematopoietic Stem Cell Transplant in case of malignant haematological disease or solid organ transplant in case of terminal stage organ failure, transplantation can be rendered hazardous by an inappropriate immune response due to lack of tolerance toward graft tissue. Indeed, Host versus Graft disease can sometimes be fatal despite immunosuppressive therapy. The latter can also be quite debilitating for patients, putting them in high risks of infection and tumorigenesis. This lack of tolerance towards grafts could be overcome by the use of Tregs.
Recently in cardiology, administering Tregs to mice with myocardial infarction reduced left ventricular remodelling that favors the evolution towards fatal heart failure.
Furthermore, our tolerogenic Tregs can bestow their capable forces when unnecessary, preventing thus the activation of proper immune effectors and delaying the generation of a required immune response. This is especially seen in infants who have a high Tregs-to-Teffectors ratio because of their exposure during their fetal life to tolerogenic molecules.
To further explain this idea, let us remind that in a mother’s organism, tolerance is necessary in order not to reject her fetus who by the way is a melange of maternal and paternal antigens (very well in the beginning when spermatozoid and oocyte fuse). The maternal-fetal interface where mother and fetus are in close contact would be the place of high tolerogenic activity. Otherwise an abortion would happen. Therefore, the fetus would be born having high concentrations of tolerance-inducing cytokines. This renders vaccination in the early life of an infant very challenging because some vaccines would be
In oncology too, a harmful tolerance towards the tumor can be induced which is a predictor of bad outcome. In fact, the Tumor Micro-Environment is very rich in cytokines and chemotactic molecules that induce a regulating function in naïve T cells, attract nTregs and favors their stability. In these two instances, checkpoint inhibitors and other immunotherapies based on the understanding of Tregs mechanisms of action have been proved efficient (cf. Afir’s article). They will be discussed in detail further in this issue.
We will mainly talk about Tregs cell-based therapy in type-1 diabetes mellitus (T1DM) and Host versus Graft Disease (HvGD).
Type 1 Diabetes mellitus: thanks to NOD mice, to which the transfer of Tregs
Furthermore, their surface markers aren’t unique to them, including CD25, and their specific marker Fox P3 can be expressed transiently by other T cells which could cause impurities during isolation and transfer (transferring T effectors could exacerbate inflammation and influence
Additionally, few centres throughout the world have mastered biological methods of isolating pure Tregs and expanding them ex-vivo.
Once expanded, these iTregs could be unstable conferring the risk of losing modulatory function once inside the host. This is often palliated by using IL2 based therapy. IL2 has a dual function: it can have a stimulating and an inhibiting effect. By binding to its low-affinity receptor CD122 present on top of NKs and CD8+ T cells, it exerts a pro-inflammatory function. However, it can bind to CD25 with a high affinity and stimulate Tregs’ modulatory function. Therefore, high doses of IL2 will activate CD122 and stimulate immune response while low doses will mostly activate CD25 and
Others are testing the co-administration of tolerogenic peptides with Tregs. These peptides are preferably autoantigens that would act as a vaccine stimulating autoantigen-specific Tregs. However, these peptides might activate Teffs and could accelerate the evolution of the disease even more. Difficulties also lie in the choice of the autoantigen. Which would be tolerogenic and which wouldn’t?
Finally, the use of probiotics (i.e. live microorganisms which when administered or consumed as part of food in adequate amounts confer a health benefit on the host) has been shown to slow the evolution of T1DM and minimize pancreatic islets destruction. This is thought to cause intestinal generation of Tregs which would travel peripherally and inhibit diabetogenic T cells. Using this method could be beneficial in producing and expanding Treg specific populations or in enhancing injected Tregs function.
Transplantation: inhibiting HvGD through administration of Tregs is likewise being investigated. It faces the same difficulties in manufacturing as well as a couple more.
The particular timing of administration, the dose administered and the evaluation of the outcome are still to be determined and optimized.
To counteract the manufacturing difficulties, some trials have tested the use of umbilical chord blood as a source of potential Tregs (again tolerance-favouring milieu in maternal-fetal interface).
Another challenge would be of an ethical order: administering a therapy that
And finally, this operation must prove costly (estimated to 40000€ or 5410000DA per patient in the ONE study) and unless it would prevent definitely the use of immunosuppressive therapy, HvGD and its complication and overall medical costs that are estimated to 6750€ or 913000DA per person annually, its high cost might limit its usage.
In conclusion, thanks to the discovery of Tregs by Sa
Références :
1- Choo E, Lee J, Park E, Park H, Jung N, Kim T & al. Infarcted Myocardium-Primed Dendritic Cells Improve Remodeling and Cardiac Function After Myocardial Infarction by Modulating the Regulatory T Cell and Macrophage Polarization. Circulation. 2017;135(15):1444-1457.
2- Gliwiński M, Iwaszkiewicz-Grześ D, Trzonkowski P. Cell-Based Therapies with T Regulatory Cells. BioDrugs. 2017;31(4):335-347.
3- Janson P, Winerdal M, Marits P, Thörn M, Ohlsson R, Winq- vist O. FOXP3 Promoter Demethylation Reveals the Committed Treg Population in Humans. PLoS ONE. 2008;3(2):e1612.
4- Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N et al. Health Benefits of Probiotics: A Review. ISRN Nutrition. 2013;2013:1-7.
5- Mahr B, Unger L, Hock K, Pilat N, Baranyi U, Schwarz C et al. IL-2 / a-IL-2 Complex Treatment Cannot Be Substituted for the Adoptive Transfer of Regulatory T cells to Promote Bone Marrow Engraftment. PLOS ONE. 2016;11(1):e0146245.
6- Ndure J, Flanagan K. Targeting regulatory T cells to improve vaccine immunogenicity in early life. Frontiers in Microbiology. 2014;5.
7- Owen J, Punt J, Stranford S, Jones P. Kuby immunology. 7th ed. New York: W.H. Freeman and Company; 2013.
8- Romano M, Tung S, Smyth L, Lombardi G. Treg therapy in transplantation: a general overview. Transplant International. 2017;30(8):745-753. (see tables of ongoing trials using Tregs in transplantation in the end of this article)
9- Sakaguchi S, Vignali D, Rudensky A, Niec R, Waldmann H. The plasticity and stability of regulatory T cells. Nature Reviews Immunology. 2013;13(6):461-467.
10- Sakaguchi S1, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995 Aug 1;155(3):1151-64.
11- Speiser D, Ho P, Verdeil G. Regulatory circuits of T cell function in cancer. Nature Reviews Immunology. 2016;16(10):599-611.
12- Webb G, Hirschfield G, Lane P. OX40, OX40L and Autoimmunity: a Comprehensive Review. Clinical Reviews in Allergy & Immunology. 2015;50(3):312-332.
13- Yu H, Paiva R, Flavell R. Harnessing the power of regulatory T-cells to control autoimmune diabetes: overview and perspective. Immunology. 2017;153(2):161-170.
14- Zhang D, Tu E, Kasagi S, Zanvit P, Chen Q, Chen W. Manipulating regulatory T cells: a promising strategy to treat autoimmunity. Immunotherapy. 2015;7(11):1201-1211.